Blog
SFDA Approves Breakthrough Biotech Diagnostic Test for Early Alzheimer’s Detection


According to an official statement released today, the newly authorized test delivers results in under 20 minutes by detecting the concentration of the pTau181 protein in a patient’s blood plasma. This marks a major advancement over traditional diagnostic methods, which often require invasive cerebrospinal fluid (CSF) analysis or costly positron emission tomography (PET) scans.
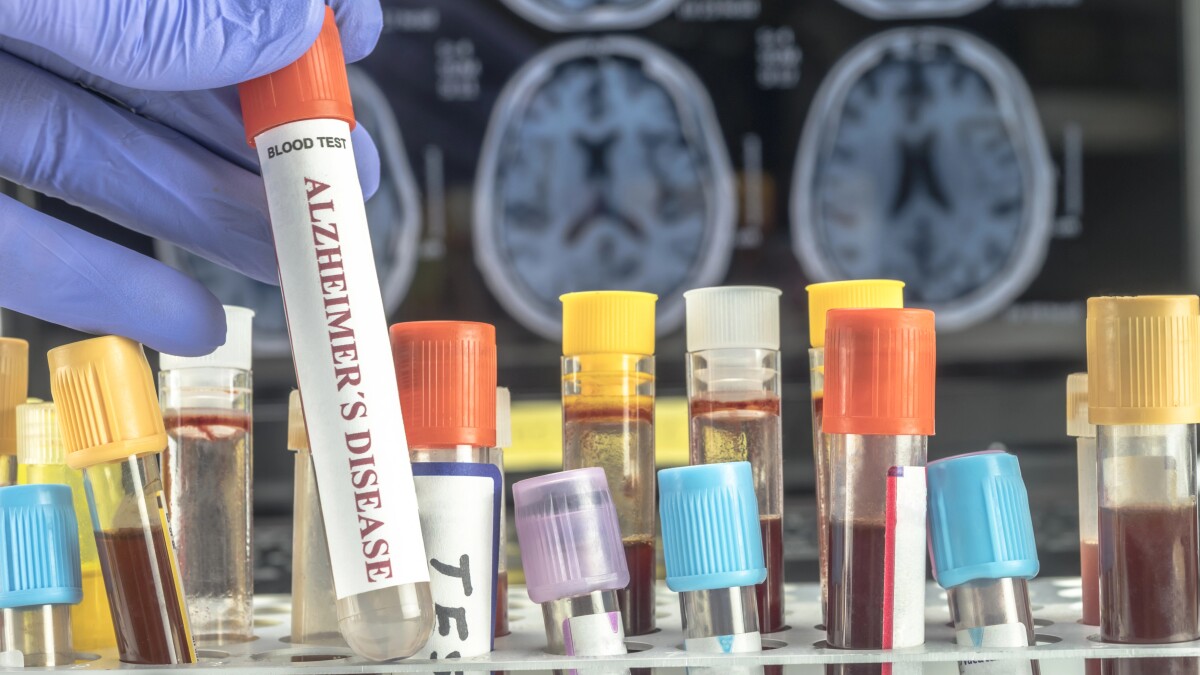
The SFDA confirmed that marketing authorization followed a rigorous scientific evaluation conducted by its experts. The review included a thorough analysis of the device’s technical and clinical documentation, along with clinical trial data, to validate its safety, efficacy, reliability, and diagnostic accuracy.
This approval supports the SFDA’s commitment to advancing the National Biotechnology Strategy and the Health Sector Transformation Program—two pillars of Saudi Vision 2030.
Related Topics :
SFDA Reports Record 82% Surge in Licensed Factories, Warehouses in 2024
Is wheat harmful to the intestine? SFDA clarifies the truth
New Corona vaccines to arrive at the Kingdom: SFDA
SFDA: National Biotechnology Strategy Highlights Saudi Leadership in Medical Innovation
Short link :
Post Views: 25